Summary of findings of the Dementia Trial
(Texas, USA)
Double blind placebo controlled trial assessing the efficacy of the CognitoLite cranial phototherapy device.
Results
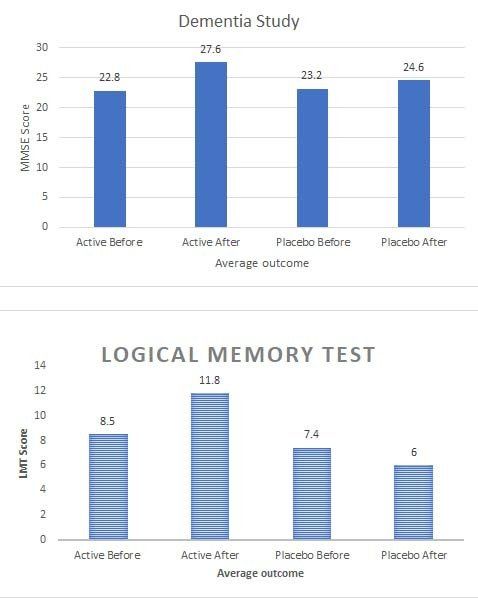
Mini Mental State Exam (MMSE). In the active arm, the average MMSE score improved from 22.8 +/- 2.6 at the beginning of treatment to 27.6 +/- 2.8 ( p < .001) at the end of the treatment, which was 4.8 points improvement (21.0% increase) over the course of treatment. In the control arm, the average MMSE score changed from 23. 2 +/- 1.6 at the beginning of treatment to 24.6 +/- 2.5 ( p = .066) at the end of the treatment, which was 1.4 points improvement (6.2% increase) over the course of treatment. The outcome assessment did identify there was a small placebo effect, however the active Cognitolite demonstrated a significant improvement compared to placebo.
Trail Making Test A. Positive results demonstrated by patients treated with active tNIR light device. They could perform noticeably faster and stayed focused on given task without being distracted or reminded with instructions. This test resulted in increased speed of performance, and the average time to completion of task decreased from 67.8 +/- 36.5 seconds at the beginning of the treatment to 51.8 +/- 22.7 seconds ( p = .03) at the end of the treatment which was 16.0 seconds (23.5%) faster than the average performance before the treatment. This finding is in stark contrast to sham treated patients’ performance which demonstrated a broad range of responses from no change to slower performance. Average time to completion of sham treated dyad changed from 68.4 +/- 35.5 seconds at the beginning of treatment to 70.6 +/- 41.4 seconds ( p = .87) at the end of treatment. This resulted in average of 2.2 seconds (6%) slower performance for the task when compared to results before sham treatment .
Trail Making Test B. More comprehensive and demanding in execution than Test A, Test B utilizes the same cognitive skills as Test A, but incorporates a mental flexibility component, and is given twice as much time for execution. Study patients undergoing active tNIR light treatment could successfully replicate Test A trends on this test. The average time to completion of task decreased from 170.3 +/- 82.7 seconds at the beginning to 129.9 +/- 55.3 seconds ( p = .03) at the end of treatment, which is 40.4 sec (23.7%) faster performance compared to the speed of execution prior to the treatment. On other hand, sham treated patients, once again, demonstrated trend of slower execution after completion of treatment course. In this group, an average time of completion changed from 167.9 +/- 80.8 seconds to 176.6 +/- 88.0 seconds ( p = .80) resulted in slower performance by 8.7 sec (5.2%) when compared with time of performance before the Boston Naming Test (BNT). Patients treated with active device had significantly better average performance on BNT compared to sham treated group. Average score in this group improved from 24.4 +/- 4.4 at the beginning
to 26.6 +/- 3.8 ( p = .02) at the end of treatment, which is 2.2 points increase (8.8%) compared to levels before treatment. Some responses to the treatment resulted in up to 35.3% of score improvement. These results were noted in three cases in patients diagnosed with moderate dementia. Sham treated patient s demonstrated insignificant BNT average score change from 23.4 +/- 4.6 to 24.3 +/- 4.9 ( p = .58), which is 0.9 points shift (4.0%) compared to the level before treatment.
Auditory Verbal Learning Test
Immediate (A.V.L.T. 1). Treatment with active device resulted in statistically significant positive cognitive improvement in patients’ performance during the course of treatment. Immediate A.V.L.T. 1 test resulted in improvement of evaluated performance for Trial 1 by 48.6% ( p < .001), Trial 5 by 31.2%
(p = .001), Trial 1 5 Sum by 33.9% ( p < .001), and Trial 7 by 47.7% ( p = .002). On other hand, sham treated patients did not demonstrate statistically significant results in the end of the treatment. In most subtests, response to placebo treatment had no change in
performance (for Trial 1 and Trial 5) or insignificant trend (Trial 1 5 Sum) of improvement.
Delayed (A.V.L.T. 2). Delayed (30 min) recall and recognition subtests were evaluated in patie nts from both groups. Significant improvement was noted in the active group in delayed recall subtest with an average score improvement by 2.2 (63.5%) points increasing from 3.4 2.9 to 5.6 4.4 ( p = .015) over the course of treatment.
Significant observation was also noted for recognition subtest with performance improvement by 14.5% ( p = .05) compared to score at the beginning of the treatment. However, sham treated patients did not demonstrate significant improvements on aforementioned subtests.
After the treatment with the Cognitolite was stopped the participants deteriorated indicating that ongoing application of the CognitoLite was necessary to maintain these cognitive gains.
Caregiver Feedback
Our study also demonstrated improvement of sleep in active group compared to feedback from patients and family caregivers in the placebo group. Although without any specific measurement, it carries scientific merit and should be integrated into future studies given the important role of long and uninterrupted sleep in dementia and elderly population, as sleep disturbance has been well demonstrated in dementia patients . Caregiver notes and patient feedback indicated that patients treated with active tNIR light devices have improved night sleep with an average of 1 hour longer after 6-10 days of treatment. This was not reported in the control group. Patients in the active group also reported decreased episodes of recurrent nightmares they had for years before the study. These feedbacks are indicative of improved overall duration of night sleep which is impaired in patients with dementia .
This improvement alone could contribute to a better recovery. Other important findings were positive changes in daily routine, improved mood, less anxiety, more energy and engagement after approximately 14 to 21 days of treatment. These observations were shared by both the patients and family members. Great importance noted by many caregivers was improved patient’s engagement in daily living, helping around the household, remembering instructions and participation in activities.